Continuing Professional Development (CPD) Activities Conflict of Interest Declaration and Management
Title: Continuing Professional Development (CPD) Activities Conflict of Interest Declaration and Management
Original Date: November 2017
Effective Date: 14 June 2023
Identification Number: OP 4043
Last Review/ Revision Date: May 2023
Next Review Date: May 2026
HMC Facilities: All HMC Hospitals / Entities
-
1.0 POLICY STATEMENT AND PURPOSE:
-
1.1 This policy is formulated to guide all Hamad Medical Corporation (HMC) healthcare practitioners on the process of declaration and management of conflict of interest in Continuing Professional Development (CPD) activities to protect the interests of healthcare practitioners who provide and attend accredited CPD activities approved by the Ministry of Public Health, Department of Healthcare Professions (DHP) – Accreditation Section (DHP-AS).
-
2.0 DEFINITIONS:
-
2.1 Department of Healthcare Professions (DHP) at Ministry of Public Health (MOPH) – the sole authority responsible for the regulation of healthcare practitioners working in the State of Qatar.
-
2.2 Department of Healthcare Professions – Accreditation Section (DHP-AS) – is one of the three (3) main sections in the Department of Healthcare Professions (DHP) concerned with accrediting medical education activities and those which are related to different health specialties and organizing the participation of health practitioners in CPD program/activity in Qatar.
-
2.3 Accredited Continuing Professional Development (CPD) Activity – an educational activity that meets the administrative, educational, and ethical standards of the DHP-AS.
-
2.4 Continuing Professional Development (CPD) – is a holistic approach towards the enhancement of personal skills and proficiency throughout a professional’s career. It consists of educational activities that focus on discipline-specific knowledge and skills but embraces learning across a wide range of content areas and competencies (for example communication skills, professionalism, leadership and management skills, healthcare quality management, evidence based practice and clinical guidelines, information technology, collaboration and teamwork skills and quality improvement) needed to deliver high-quality healthcare. The CPD activities are categorized as follows:
-
2.4.1 Category 1: Accredited group learning activities.
-
2.4.2 Category 2: Self-directed learning activities.
-
2.4.3 Category 3: Assessment activities.
-
2.5 HMC CPD Program Steering Committee – an HMC advisory board committee chaired by the head of Medical Education Department and comprises of members assigned by the department heads to govern, discuss, report, and evaluate the organizational agenda of the CPD events/activities at HMC and approve the CPD activities in their department.
-
2.6 Scientific Planning Committee (SPC) known as CPD Organizer – A group of people who plan, organize, and develop sponsored, funded, or non-sponsored CPD events/activities and ensure that DHP-AS accreditation standards are met.
-
2.7 Health Care Practitioners – are licensed practitioners by Department of Healthcare Professions (DHP) at Ministry of Public Health (MOPH) in the state of Qatar which includes physician, dentist, nurse, midwife, pharmacist, allied (e.g., dietician, respiratory therapist, physical therapist, technologist, paramedics) and others.
-
2.8 CPD Activities Conflict of Interest – A set of conditions that may occur in situations where the individual or family member’s personal and professional interests may have actual, potential, or apparent influence with their duties or responsibilities in providing or contributing in CPD activities.
-
2.9 Interested Persons – An individual in a position to control the content of an educational activity, including the chair and members of the scientific planning committee (SPC) speakers, educators, training providers, authors, content developer, moderators and facilitators.
-
2.10 Sponsor(s) – An individual, group, commercial company, corporation, organization, institution (profit and nonprofit) that contribute financial and in-kind support such as tools or services or human resources which have a monetary value (e.g., giveaways, gifts, prizes, or catering services) to an accredited CPD activities.
-
3.0 RESPONSIBILITIES:
-
3.1 Director of Medical Education / CPD Chair: Responsible for approving this policy.
-
3.2 HMC CPD Program Steering Committee: Responsible for developing, revising, updating & disseminating this policy.
-
3.3 Department CPD Lead: Responsible for educating, implementing and monitoring the compliance to this policy.
-
3.4 The Scientific Planning Committee: Responsible for managing the declared conflict of interest.
-
3.5 Speakers, educators and training providers, authors, content developer, moderators, facilitators and interested persons including the Department head/Chairman: Responsible for declaring the conflict of interest and comply with the policy.
-
3.6 All HMC Health Care Practitioners: Responsible to be aware and comply with this policy.
-
4.0 PROCEDURE/PROCESS:
-
4.1 All HMC healthcare practitioners are expected to demonstrate their commitment to maintaining their competence and enhancing their performance by participating in Continuing Professional Development (CPD) throughout their careers, a two (2) year CPD cycle as per DHP-AS requirements.
-
4.2 The HMC accredited CPD activities shall be based on the identified educational learning needs, new technologies, best practices in healthcare including the national and organizational healthcare vison and priorities.
-
4.3 The SPC, speaker and presenter should declare the conflict of interest in every CPD activities to:
-
4.3.1 Assure learners that they are not inadvertently exposed to promotion, financial interest, marketing, and commercial bias.
-
4.3.2 Indicate that the content provides a scientifically credible evidences, valid, balanced, and current best practices information.
-
4.4 Sponsor(s), sponsors representative, organizations hired by sponsors and commercial interest should never have direct or indirect involvement or influence in any aspect of development, delivery, or evaluation of accredited CPD activity.
-
4.5 The terms, conditions and purpose of the sponsorship/support provided must be documented in a written agreement signed by the Sponsor and SPC Chair or authorized HMC designee (refer to policy OP 4041, Sponsorship of Accredited CPD activities).
-
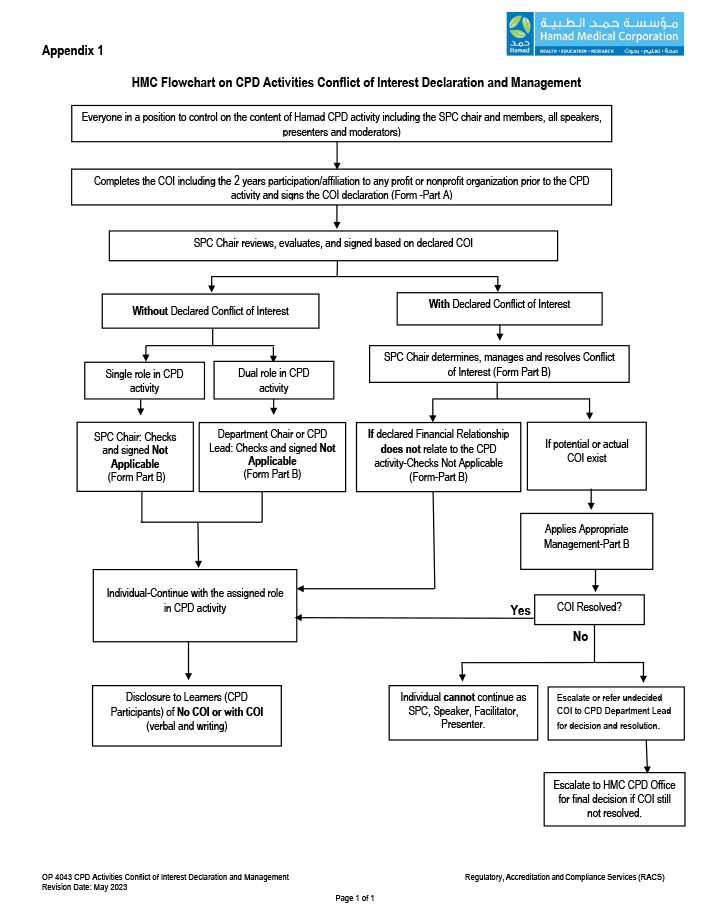
4.6 The full process of declaration and management of conflict of interest shall follow the attached flowchart. (Refer to Appendix1, HMC Flowchart on CPD Conflict of Interest Declaration and Management)
-
4.7 The Scientific Planning Committee shall:
-
4.7.1 Oversee the accredited CDP activities:
-
4.7.1.1 Category 1:
-
4.7.1.1.1 Educational Rounds and Journal Clubs, three (3) years from the start date of accreditation and shall be reviewed accordingly.
-
4.7.1.1.2 Conference, symposia, seminar, workshops, online synchronous and blended learning activities, one (1) year from the start date of the accredited CPD activity.
-
4.7.1.2 Category 3: Assessment Activities (e.g., knowledge assessment programs, simulation, direct observation of procedures or performance in practice, clinical audits, multi-source feedback) shall be for a maximum of three (3) years from the start date of accreditation and shall be reviewed accordingly.
-
4.7.1.3 Combined Category 1 & 3: Conferences, symposia, seminar, workshops, online synchronous and blended learning and assessment activities, shall be maximum of one (1) year from the start date of the accredited CPD activity.
-
4.7.2 Control the content of the Hamad CPD educational activities with roles and responsibilities stated in OP 4041 Sponsorship of Accredited CPD activities.
-
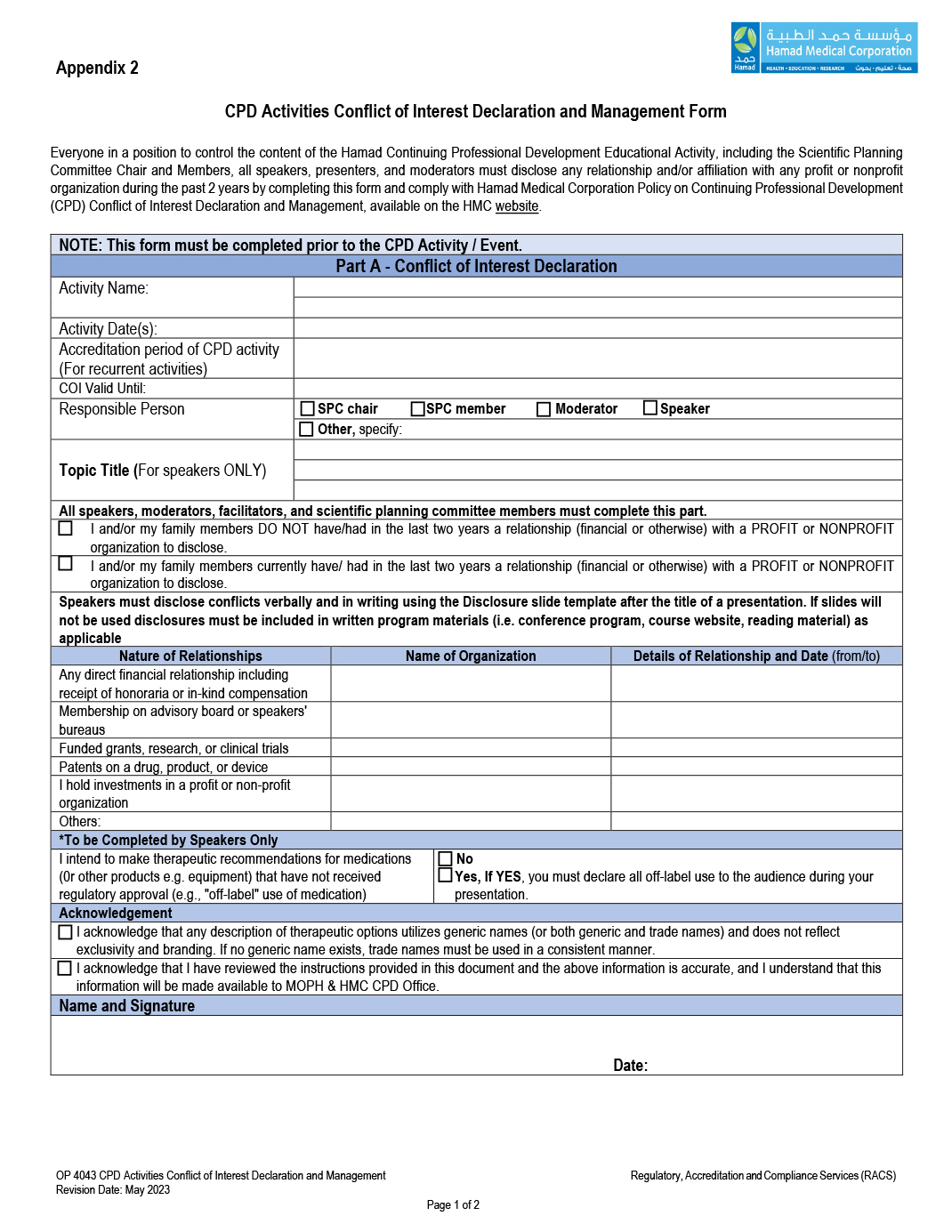
4.7.3 Ensure that the form is completed (Refer to Appendix 2, Conflict of Interest Declaration and Management form, Part A) and signed even there is no actual or potential conflict of interest two (2) years prior to CPD activity including any affiliation or relationship to external organization.
-
4.7.4 Compile all conflict of interest and other required CPD documentation, store in compliance with DHP-AS standards for six (6) years and be readily available for audit purposes if required.
-
4.7.5 Not accept advice from a sponsor on condition of receiving financial or in-kind support.
-
4.8 Training on the use of existing product or medical device / equipment shall be approved as an accredited CPD activity ONLY if it fulfils the following criteria:
-
4.8.1 The activity is organized by HMC CPD Department(s), or the SPC/CPD organizer.
-
4.8.2 The activity is not for marketing purposes and does not include promotional materials.
-
4.8.3 The materials, products or devices are already utilized in the care of patients within HMC clinical area / department.
-
4.8.4 The participation of the representative of the commercial organization related to company product or services is approved and rationalized by the SPC.
-
4.8.5 The representatives of the commercial company should not act as educators/speakers/facilitators during educational sessions unless they are providing an essential demonstration and when no healthcare practitioner is qualified to provide such demonstration.
-
4.9 The interested person including the speakers, educators, training providers, authors, content developer, moderators and facilitators must:
-
4.9.1 Complete and sign the Conflict of Interest Declaration and Management form, Part A. (Refer to Appendix 2)
-
4.9.2 Disclose the existence of any financial and in-kind support or affiliation (profit or non-profit) including those for the past two (2) years prior to the CPD activity date.
-
4.10 Scientific Planning Committee Chair shall:
-
4.10.1 Explain to everyone involved in the CPD activity that any undisclosed Conflict of Interest shall be considered a violation of ethical standards.
-
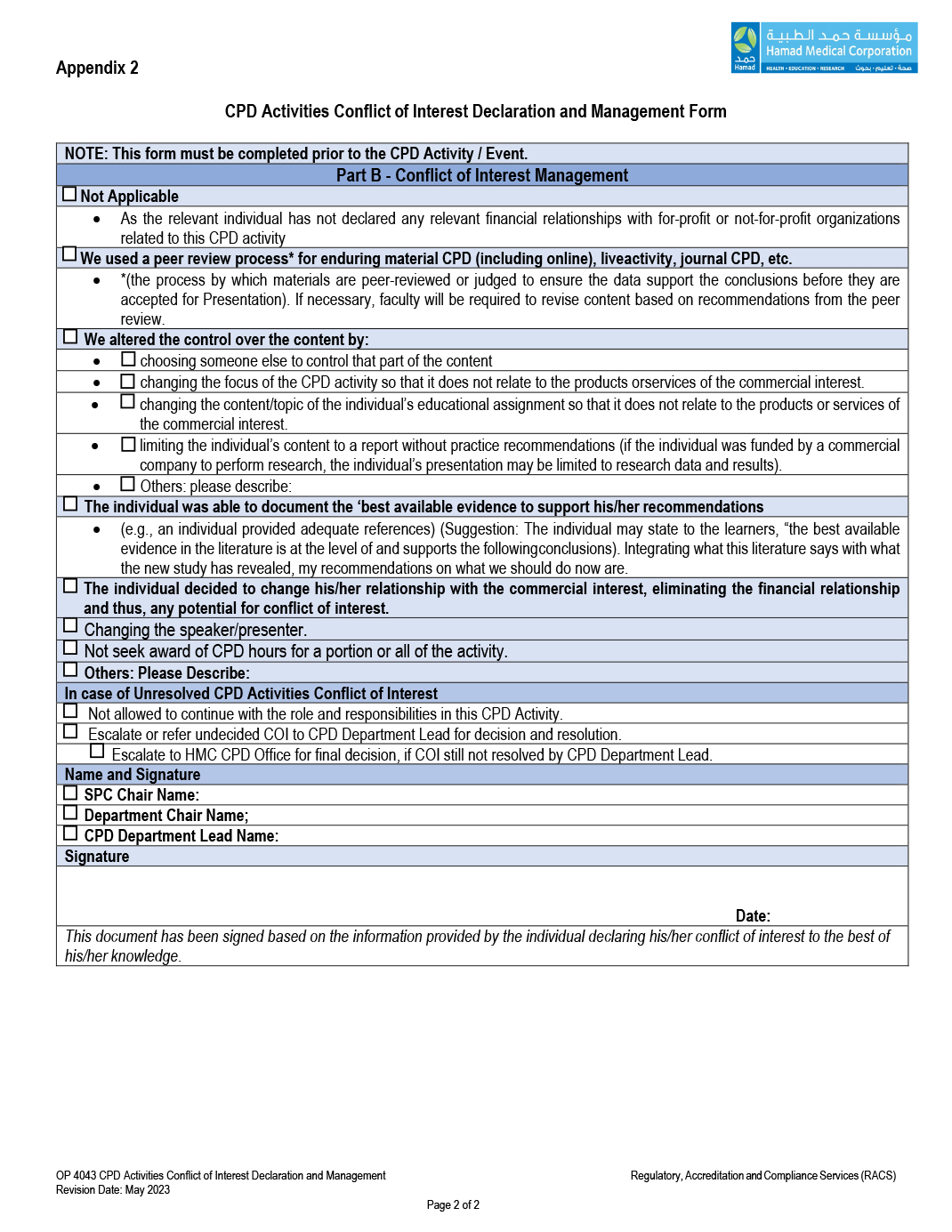
4.10.2 Review the submitted form (Part A) of the SPC members, speakers, presenters, facilitators, moderators, faculty even in cases where there is nothing to disclose and complete Part B of the form.
-
4.10.3 Identify, determine, and manage the actual and/or potential conflicts of interest.
-
4.10.4 Responsible for reviewing any responses that raise concerns regarding bias and report it to HMC CPD Office via the Chair of the relevant Department CPD Committee/CPD Lead.
-
4.11 The Part B of the form shall be signed by the Chairman/Head of the department or the CPD department lead for:
-
4.11.1 SPC chair.
-
4.11.2 SPC member with additional role like speaker or moderator.
-
4.12 Organizational affiliation that were not written in the form, Part A, that can result to potential or actual Conflict of Interest are as follows:
-
4.12.1 Ownership or investment interest in any commercial entity (pharmaceutical company, medical devices manufacturer, nutritional support, communication, and event planning company.
-
4.12.2 Involvement of design of clinical studies using products mentioned in the educational activity.
-
4.12.3 Ownership of patent of a commercial product referred to in the presentation.
-
4.13 Managing Conflict of Interest by the SPC:
-
4.13.1 Any identified conflict of interest and its resolution should be managed first by SPC chair and members.
-
4.13.2 The Scientific Planning Committee may apply the recommendation in the form Part B.
-
4.13.3 If the SPC Chair and/or members of the Scientific Planning Committee are identified as an interested person, they must exempt themselves from any discussion, decision-making, arrangement and management of the Conflict of Interest.
-
4.13.4 In case of unresolved actual or potential conflict of interest, the SPC shall either:
-
4.13.4.1 Decide to forego or not allow the interested persons to continue the assigned role and responsibilities pertaining to the CPD activity.
-
4.13.4.2 Or, refer to the Departmental CPD lead for further deliberation if necessary.
-
4.14 Disclosure of Conflict of Interest to Participants (Learners)
-
4.14.1 Disclosure of conflict of interest in a presentation must be:
-
4.14.1.1 In verbal and visual.
-
4.14.1.2 Displayed on a slide after the title of the presentation. (Refer to Appendix 3, Disclosure Statement Slide).
-
4.14.1.3 Another slide shall be required for the nature and names of any commercial relationship, the support received and how potential bias has been mitigated and/or managed in case of actual or potential conflict of interest:
-
4.14.1.3.1 If the presentation includes therapeutic recommendations for medications that have not received regulatory approval (e.g., “off-label medication”) must be disclosed.
-
4.14.2 The written educational materials (e.g. course handouts, program syllabus or program brochure) shall have this disclosure statement “The scientific planning committee has reviewed all disclosure financial relationships of speakers, moderators, facilitators and/or authors in advance of this CPD activity and had implemented procedures to manage any potential or real conflicts of interest”.
-
4.14.3 Further guidance on disclosure of relevant financial relationships is provided in Appendix 4.
-
4.15 All participants of the accredited CPD activities should have the opportunity to complete an evaluation form that allows them to:
-
4.15.1 Identify whether the overall and individual session learning objective were met.
-
4.15.2 Identify the potential impact of the activity for their practice.
-
4.15.3 Identify whether the content was balanced and free or commercial or other sources of bias.
-
4.15.4 Ensure whether member of the SPC, speakers, moderators, facilitators and/or authors disclosed their relationships.
-
4.15.5 Indicate if they perceive bias in a presentation and/or activity.
-
4.15.6 Check if the speaker or presenter declare a Conflict of interest in the presentation slide.
-
4.16 Managing Unresolvable Conflict of Interest by HMC CPD Office
-
4.16.1 The HMC CPD office shall:
-
4.16.1.1 Review CPD activities with unresolved conflict of interest, forwarded by the Department CPD Lead and responses from participants that raise concerns about bias.
-
4.16.1.2 Retain and maintain a record of all deliberations, decisions, actions taken and made it available for audit purposes to the DHP-AS.
-
4.16.1.3 Inform in writing the relevant department CPD Lead and Scientific Planning committee Chair of any course taken following deliberation of a conflict of interest or perceived bias.
-
5.0 DOCUMENTATION:
-
5.1 Any interested person who wants to apply for CPD activities shall complete the attached form (Appendix 2).
-
5.2 The disclosure statement slide of conflict of interest (Appendix 3) must be presented in every CPD activities.
-
6.0 REFERENCES:
-
6.1 DHP-AS Ethical Standards for Accredited CPD Activities accessed through The National System for Continuing Professional Development, Qatar (moph.gov.qa).
-
6.2 Royal College of Physicians and Surgeons of Canada, The College of Family Physicians of Canada, College Des Medecins Du Quebec (2017) available for download from National Standard for Support of Accredited CPD Activities: The Royal College of Physicians and Surgeons of Canada.
-
6.3 OP 4011 Conflict of Interest.
-
6.4 Joint Commission International Accreditation Standards for Hospitals, 7th Edition.
-
7.0 ATTACHMENTS:
-
7.1 Appendix 1: HMC Flowchart on CPD Activities Conflict of Interest Declaration and Management.
-
7.2 Appendix 2: CPD Activities Conflict of Interest Declaration and Management Form.
-
7.3 Appendix 3: Disclosure Statement Slide.
-
7.4 Appendix 4: Guidance on Disclosure of Relevant Financial Relationships.
-
8.0 TRACKING HISTORY OF CHANGES:
Revision
Date |
Version
Number |
Section Number
|
Summary of Changes
|
| May 2023 |
2.0 |
Header Section
|
• Version replaced with Effective Date
• Last Revision Date added with the Review
• The word Changes was added to Tracking
History
• Hospital(s) replaced by HMC Facilities
• Policy Title revised and modified.
|
| |
|
1.1
|
• Statement and Purpose section revised and modified with new information added.
|
| |
|
2.1, 2.2, 2.3, 2.4,
2.5 & 2.7
|
• New definitions added.
|
| |
|
2.6, 2.8, 2.9 & 2.10
|
• Definitions revised and modified with new information added.
|
| |
|
3.0
|
•
Responsibilities: new section added to the policy with new information from section 3.1 to 3.6
•
Change in numbering of policy content.
|
| |
|
4.0
|
• Major changes in the Procedure / process section.
• New information added to clarify all the processes of CPD activities conflict of interest.
|
| |
|
5.1 & 5.2
|
• New information added.
|
| |
|
6.1 & 6.2
|
• Websites of the references updated.
|
| |
|
6.4
|
• New reference added.
|
| |
|
7.1, 7.2 & 7.3
|
• Attachments titles and contents revised and modified.
|
| |
|
7.4
|
• Change the term QCHP to DHP-AS.
|
|
Subject Matter Expert
|
• HMC CPD Program Steering Committee. Contributors:
|

Appendix 4
Guidance on Disclosure of Relevant Financial Relationships
- 1. Hamad CPD must ensure balance, independence, objectivity, and scientific rigor in educational activities. All individuals in a position to control educational content must disclose the name of commercial interests producing, marketing, or distributing healthcare related goods or services with which the individual has had a relevant financial relationship within the past 24 months.
- 2. Please review the definitions of “commercial interest”, “financial relationships”, “relevant financial relationships”, and “conflict of interest” to ensure compliance.
- 3. Definitions:
- 3.1 Commercial interest is any entity producing, marketing, or distributing healthcare related goods or services used on patients. Clinical service providers are not considered commercial interests.
- 3.2 Financial relationships are those in which an individual benefits by receiving a salary, royalty, consulting fee, honoraria, ownership interest (e.g., stocks), or other financial benefit, usually associated with roles such as employment, independent contractor (including contracted research), consulting, speaking and teaching, membership of advisory committees, review panels, or board membership, and other activities for which remuneration is received, or expected. Relationships of the individual’s spouse are included as those of the individual.
- 3.3 Relevant financial relationships with commercial interests are any that occurred in the 24 months preceding the time the individual is involved in controlling educational content. There is no minimum payment for relationships to be considered relevant. Inherent in any amount is incentive to maintain or increase the value of the relationship.
- 3.4 Conflict of Interest: Circumstances create a conflict of interest when an individual has opportunity to affect educational or scientific content about products or services of a commercial interest with which he/she has a relevant financial relationship.
- 3.5 Required Disclosure of Relevant Financial Relationships during Educational Presentations:
- 3.5.1 At the beginning of an educational presentation, both SPC members and all contributing faculty must disclose all relevant financial relationships.
- 3.5.2 Speakers with no industry involvement should inform the audience that they have no conflict of interest.
- 3.5.3 Financial relationships entirely unrelated to the topic do NOT need to be disclosed.
- 3.6 Any involvement should be declared with a suitable phrase e.g.
- 3.6.1 “I have/had an affiliation (state financial or otherwise) with X pharmaceutical (or medical device etc.) organization.”
- 3.6.2 “I am a member of an Advisory Board/ Speakers Bureau (or equivalent) with X organization.”
- 3.6.3 “I have received payment from X organization (including gifts or 'in kind' compensation).”
- 3.6.4 “I have received grant(s)/ an honorarium from X organization.”
- 3.6.5 “I hold a patent for a product referred to in the CPD activity (or that is marketed by X organization).”
- 3.6.6 “I hold investments in X organization.”
- 3.6.7 “I am currently participating in (or have participated in) a clinical trial within the past two years.”
- 3.6.8 If relevant financial relationships do exist, in addition to disclosure, faculty must declare how the content has been adjusted to avoid commercial bias. For example, faculty may state that:
- 3.6.8.1 “I am covering topics other than those represented by my relationship with (Name of commercial entity).” Or
- 3.6.8.2 “Any recommendations made during this presentation are evidence-based, or consistent with current consensus-based practice.” Or
- 3.6.8.3 “I will not be presenting this content in a promotional manner.” Or
- 3.6.8.4 “I will not endorse (name of commercial entity) during this presentation.”
- 3.7 Promotional Activity Restriction & Non-endorsement of Commercial Entities Endorsement of commercial entities, products, goods and services is not permitted in DHP-AS accredited learning sessions. This will be strictly enforced.
