COMMERCIAL SPONSORSHIP
COMMERCIAL SPONSORSHIP: COMPLIANCE WITH DHP ETHICAL STANDARDS
Policy OP 4041: Sponsorship from Commercial Sources of Accredited Continuing Professional Development
(CPD) Activities
You must ensure that your arrangements with any commercial sponsor comply with the points below to be eligible for DHP CPD. If you need clarification please get in touch with your relevant CPD lead. All this information is within the DHP Ethical Standards.
Corporate Communications have been asked to be very careful, but other conference companies will need particular guidance.
That is your responsibility as SPC.
Commercial Sponsors are permitted but
must be disclosed to your audience and acknowledged with the DHPs approved statement. E.g. with a slide at the start of meeting
using
this statement
“This CPD activity is supported by financial and/or in-kind support from the following sponsors:”
Use company names
not logos
Beyond the standard acknowledgement statement of financial and in-kind support the linking of a sponsor’s name (or other branding) to any educational session or section of an educational program within an accredited group learning activity
is prohibited.
Sponsorship recognition must appear on a page separate from the educational content, activity schedule,
learning objectives, and accreditation statement i.e. on a separate (usually back) page of any program or other publicity material -
never on the front page.
Ideally use a completely separate sponsorship acknowledgment booklet, which ONLY details sponsorship, then you may use logos.
Product-specific advertising, promotional materials or branding strategies cannot be included on/appear within locations where accredited CPD sessions are occurring (e.g. lecture halls, small group discussion rooms) immediately before, during or immediately after an accredited CPD activity
This means any flyers/ roll ups etc. with logos on must be well
outside the room(s) where the accredited activity takes place - usually we advise in the coffee or eating room.
The rollups should be only about thanking them for the sponsorship - not linking the company logos to the event title or any other activity information.
Product-specific advertising, promotional materials or branding strategies
cannot be included on, appear within, or adjacent to:
- Any educational materials, slides, abstracts and handouts used as part of an accredited CPD activity
- Activity agendas, programs or calendars of events (preliminary and final)
-
Any webpages or electronic media containing educational material. -
Maybe use a hyperlink to the
sponsor’s page?
Commercial exhibits or advertisements must be arranged in a location that is completely separated from the accredited CPD activity.
Sponsors must have no influence (direct or indirect) over meeting content. The SPC cannot be required to accept advice concerning speakers, the activity development, delivery or evaluation as a condition of sponsorship.
The terms, conditions and purposes by which sponsorship is provided must be documented in a written agreement that is signed by the SPC and sponsor (you have been provided with that)
Any incentive provided to participants associated with an accredited CPD activity must be approved by the CPD provider organization. High value gifts not advised as they may be perceived as an attempt to influence. If in doubt please check with us!
If a sponsor wishes to run a satellite sponsored symposium that must be
clearly identified as such. This cannot earn CPD credit and must occur at a separate time and location that
does not compete with accredited CPD activities.
These unaccredited activities cannot be listed or included within agenda, programs or calendar of events (preliminary or final) - they must go on a completely different page, although you can indicate to the audience where to look for that information e.g. by tabling ‘lunchtime satellite symposium see next page’
All income from commercial sources must be declared as part of your full documentation of conference budget. Companies must not pay any speaker directly, neither honorarium nor expenses.
Please note: All accredited educational events are subject to both HMC and DHP team audit. For sponsored events adherence to these guidelines will be a major emphasis within the audit. Major deviation from these standards could result in your event losing its DHP CPD accreditation.
The flow diagram below outlines the essential steps in managing sponsorship of Hamad CPD Events
Completion of the sponsorship agreement below is an essential part of this process and is required for all sponsored CPD events.
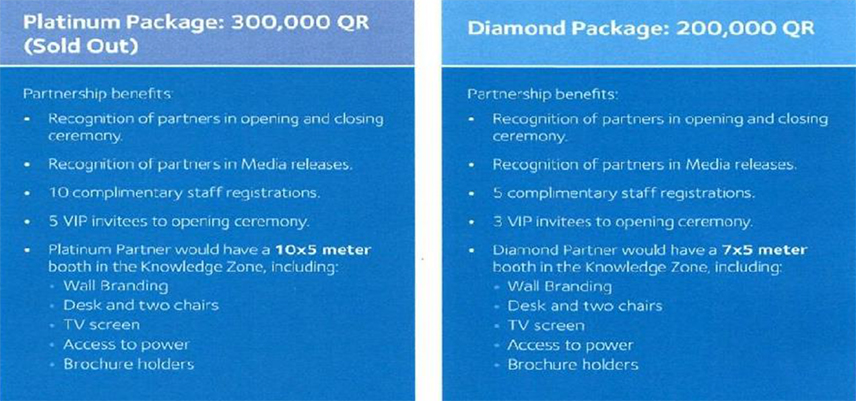
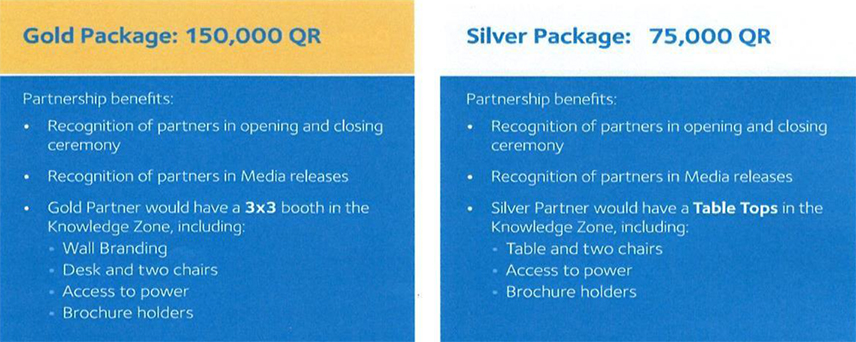
Also included is an example of a DHP compliant sponsorship prospectus.
Please note that this is probably more useful to you in terms of
what it doesn’t offer, than what it does!
OP 4041: SPONSORSHIP FROM COMMERCIALSOURCES OF ACCREDITED CONTINUING PROFESSIONAL DEVELOPMENT (CPD) ACTIVITIES
POLICY/PROCEDURE
TITLE:
SPONSORSHIP FROM COMMERCIAL SOURCES OF ACCREDITED CONTINUING PROFESSIONAL DEVELOPMENT (CPD) ACTIVITIES
ORIGINAL DATE:
November 2017 VERSION:1.0 IDENTIFICATION NUMBER:
OP 4041
LAST REVISION DATE:
February 2018 NEXT REVIEW DATE:
February 2021
HOSPITAL(S)
ALL HMC HOSPITALS /ENTITIES
1.0 POLICY STATEMENT AND PURPOSE:
1.1 This policy outlines Hamad Medical Corporation (HMC) standards regarding the support of continuing professional development (CPD) activities or resources by commercial sponsors. Such activities or resources include but are not restricted to courses, seminars, conferences, workshops, journal clubs, hospital approved rounds, lectures, Internet courses, and the production of learning resources, e.g. CD-ROM or videotapes. Accredited activities and resources should be fair, balanced and free of commercial bias.
1.1.1 Commercial interest shall not influence:
1.1.1.1 Identification of learning needs
1.1.1.2 Development of educational objectives
1.1.1.3 Selection and presentation of content, including speakers
1.1.1.4 Selection of all persons and organizations that shall control the content of and registration of the educational activity
1.1.1.5 Selection of educational methods
1.1.1.6 Evaluation of educational activities
1.2 This policy applies to all Hamad Medical Corporation accredited CPD educational events and to entertainment, exhibits and satellite symposia occurring at any such event.
1.3 This policy is in compliance with the accreditation requirements of the Qatar Department of Healthcare Professions – Accreditation Section (DHP - AS)
2.0 DEFINITIONS:
2.1 Accreditation (applies to Continuing Professional Development courses, events,
educational resources) the successful review of an educational activity or resources by the HMC Continuing Professional Development Steering Committee (HMC CPD SC) or their delegate. Upon accreditation, HMC CPD SC may assign DHP - AS hours that shall be eligible for credits by DHP.
2.2 Accredited Provider:
2.2.1 A health professional organization or group, that plans, delivers and evaluates continuing education activities and has been recognized by an accrediting body (in Qatar the DHP - AS). This definition excludes pharmaceutical companies and their advisory groups, medical and surgical supply companies, and communication companies.
2.3 Approval:
2.3.1 For the purposes of this policy, approval implies the successful review of an educational activity or resource by a peer review and/or educational standards committee.
2.4 Continuing Professional Development (CPD) Activity
2.4.1 A CPD activity is based on identified learning needs, has a purpose or objectives, and is evaluated to assure the learning needs are met. A CPD activity is distinct from a social event.
2.5 Conflict of Interest:
2.5.1 A conflict of interest may arise where an individual’s personal or other interests are in actual, potential or perceived conflict with their duties or responsibilities to provide education or participate in an educational event.
Mere existence of a conflict of interest does not imply wrongdoing; however, when conflicts of interest do arise, they should be recognized, disclosed and properly managed. For the purpose of this document, relevant potential conflicts shall be those from the past five years.
2.6 Commercial Interest: Any entities that do business with the intent or possibility of commercial gain, generating a profit, or increasing equity. This does not include charitable organizations, military, non- governmental (NGO) or quasi-governmental organizations.
2.7 Sponsors:
2.7.1 A company, organization, institution, government agency or other entity (for -profit or not-for-profit) that contributes financial or in-kind resources to a CPD activity.
2.8 Consulting:
2.8.1 Consulting relationships include contractual relationships, advisory boards, speaker’s bureau, research and any relationship whereby the faculty member receives, or has the expectation to receive, income for services other than clinical or university work. This includes honoraria, commissioned papers, and fees for speaking, chairing and administration of meetings including in-kind considerations.
2.9 Learner (Participant):
2.9.1 Learners are participants whose learning needs have priority. Learners are responsible for identifying knowledge gaps, actively participating in filling them, and keeping track of their learning gains.
2.10 Speakers Bureau:
2.10.1 This is defined as a relationship in which the faculty member is under contract to, or paid by, a company and the company selects any of: the topic, any part of the content of a talk, or any members of the audience. Programs run by for-profit educational companies are included in this category.
2.11 Unrestricted Educational Grant:
2.11.1 All funds from a commercial source should be in the form of an educational grant payable to the institution or organization sponsoring the CPD activity, with no stipulations attached such as selecting faculty, authors, participants, or any matters related to the content. It is acceptable to designate an unrestricted educational grant to a specific CPD event. Subsidies should not be accepted if specifically designated for hospitality purposes.
2.12 Scientific Planning Committee:
2.12.1 The group of people tasked with developing the CPD activity and ensuring accreditation standards are adhered to.
2.13 Satellite Symposia:
2.13.1 These are separate meetings held in proximity (either spatially or temporally) to conferences and other CPD events. These are frequently produced by commercial interests, often without the restrictions of commercial sponsorship policies and guidelines.
3.0 PROCEDURE/PROCESS:
3.1 Separating Education from marketing:
3.1.1 Health care professionals should maintain professional autonomy and independence in relation with commercial entities (e.g. Pharmaceutical companies, instrument and device manufactures).
3.1.2 Acceptable commercial support should be distinct from activities intended to promote the marketing of a particular product.
3.2 Needs Assessment:
3.2.1 Educational events should be planned to address the educational needs of the audience, whether that be students, trainees or health care providers. Faculty planners are responsible for the content, organization and financial arrangements of these events,
without influence from sponsors.
3.2.2 A comprehensive needs assessment should be conducted, ideally using multiple means of needs assessment to determine the perceived, misperceived and unperceived needs of their learners.
3.2.3 The needs assessment should occur prior to any negotiation with potential commercial sponsors.
3.3 Course Content, Objectives and Evaluation:
3.3.1 Invitations to participate in planning for CPD activities should emanate from the CPD scientific planning committee (SPC), not from commercial sponsors. Course planners should choose course topics, learning objectives, learning methods and ensure decisions are made free of the influence of commercial interest.
3.3.2 Evaluation mechanisms should contain questions that ask whether learners perceived commercial bias in the materials received from the presenters or authors.
3.4 Selection of Topics and Speakers:
3.4.1 As a condition of receiving funds or services, a CPD provider should not be required to accept advice or services concerning the selection of teachers, authors, participants, or other education matters including content, from a commercial sponsor.
3.4.2 Travel arrangements, registration, expenses, and honoraria should all be arranged and paid through event planners, and not through commercial sponsors or their agents.
3.5 Unbiased presentation of content:
3.5.1 Presentations should give a balanced view of all available relevant therapeutic options available. In those circumstances where there is only one product, service or drug, a fair assessment should be presented to learners. The use of generic names is required. In the event trade names are employed, reference to multiple trade names representing several companies is preferable to referencing a single trade name from a single company.
3.5.2 It is prohibited to use the name or institutional logo of HMC in a manner that constitutes promotion of a commercial product (e.g. presentation slides).
3.6 Direction of Funds:
3.6.1
All funds from a commercial source should be in the form of an unrestricted educational grant to HMC. It is acceptable to designate an unrestricted educational grant to a specific CPD event.
3.6.2 Events should have sponsorship from multiple sources to avoid the perception of ownership that a single commercial source may imply. Funds should be held centrally at an institution (hospital, univers ity department or division). Funds should not be held by any one individual.
3.6.3 Audit mechanisms should be established to assure compliance with HMC and national standards. Financial statements for each sponsored event should be available for audit by the departments, HMC Department of Finance, the HMC CPD SC, and commercial sponsors.
3.7 Disclosures:
3.7.1 Disclosure of commercial affiliations, sponsorships, honoraria, monetary support, contract research, and other potential conflicts of interest should be made to the participants in a CPD activity by HMC faculty, planning committee members and visiting speakers.
3.7.2 Faculty members should fully disclose income received from participation in industry advisory boards, speakers’ bureaus or consultation to industry. Faculty disclosures should cover relevant relationships for a period of five years prior to the course. (See OP 4011 Conflict of Interest Policy).
3.8 Commercial Displays and Promotional Materials:
3.8.1
Commercial displays and materials should be in a separate room from educational activities. A statement from
the SPC to potential exhibitors and/or commercial sponsors should indicate that gift items cannot be distributed.
3.8.2 This includes small items bearing the exhibitor’s name and/or logo to the participants in the venue where the CPD activity is occurring. When commercial exhibits are included in the program, they should not influence the planning or interfere with the presentation of the educational activity.
3.8.3 Exhibitors and/or sponsors may not use Hamad Medical Corporation name or logo unless specifically approved by the HMC CPD Office.
3.9 Satellite Symposia:
3.9.1 Registrants may perceive such programs as integral to the accredited program and be unaware of commercial bias. Thus, conference planners should take care to ensure that:
3.9.1.1 Registrants at the program shall be aware that such satellite symposia are not accredited by HMC or DHP.
3.9.1.2 Such activities shall be promoted (marketed or ‘branded’) in a way which clearly identifies the satellite activity as distinct from the accredited program;
3.9.1.3 The satellite symposia shall be located in an area separate from HMC/ DHP accredited program, and.
3.9.1.4 The satellite symposia do not run concurrently with the accredited program.
3.10 Social Events:
3.10.1 Commercial sponsors may not directly subsidize or name hospitality and other arrangements for faculty, planning committees, registrants, or guests. Facilities, catering, and other activities should be in keeping with arrangements made without commercial sponsorship. These activities should not be in the control of or managed by commercial sponsors.
3.11 Registration:
3.11.1 Registration fees:
3.11.1.1 Registration for accredited programs should be through HMC faculty member planners and not through an industry representative event planner service.
3.11.1.2 A registration fee if applicable is generally required from all nonteaching participants, since it is preferable that registrants bear some responsibility for the program in order to reduce perceived or real influence on learning. Exceptions to this general rule include rounds, faculty development activities, and research-oriented programs or events.
3.11.2 Payment to Registrants:
3.11.2.1 Commercial sponsors may not provide or subsidize travel, lodging, honoraria, or personal expenses direct to practicing health professional attendees or their guests. For students, residents or fellows in accredited programs, commercial sponsorship for the participation of such learners may occur by contributing to a scholarship fund.
3.11.2.2 The selection of physician trainees and the expenditures of these funds is the responsibility of the Course Director and the relevant Department/Division Chair, Director of Postgraduate Education, or designate.
3.11.2.3 The corporate donor should always remain neutral in decisions regarding the specific allocation of such awards.
3.11.3 Sharing of Registrant Data –
Sponsors are not allowed to obtain the names or personal details of registrants from the SPC (see privacy policy).
3.12 Gifts & Payments to Teachers:
3.12.1 The planning committee may consider payment to faculty speakers for their participation at a CPD event.
At the discretion of the SPC Chair, expenses incurred in making a presentation may be reimbursed, and small gifts are acceptable.
3.13 Guest Faculty / Visiting Speakers:
3.13.1 It is appropriate for guest faculty at conferences or meetings to accept both reasonable honoraria and reimbursement for personal travel, lodging, and meal expenses. Guest faculty may not be paid directly by commercial organizations, but should be paid through the course planners. Remuneration should be commensurate with the work completed.
3.14 Acknowledgements:
3.14.1 Course directors may acknowledge commercial support as noted below. Advertising for commercial products by name or by indication is not permitted.
3.14.1.1
Course Brochures:
3.14.1.1.1
Commercial sponsors may
notbe listed in any part of: the schedule of activities or any material related to the academic content, the list of faculty (including SPC members and speakers), the course objectives, or on the front of brochures. Educational grants are documented in course brochures under “Acknowledgements.”
3.14.1.2 Posters, Flyers and One-Page Brochures:
3.14.1.2.1 Acknowledgements may be listed at the bottom in a font not larger than the text of the brochure.
3.15 Websites and other Electronic Formats:
3.15.1 In a one-page or one-screen format, acknowledgements may be listed in a font not larger than the text of the brochure. In a multi-page or multi-screen format, acknowledgements may not be on the main (home) page, on a list of faculty or with the learning activities.
3.15.2 Advertisements and promotional materials are not permitted on websites for CPD programs. “Pop-ups” are not allowed. Links should open a new window, leaving the educational site open in the background.
3.15.3 Links to commercial sponsors’ home pages (but not to pages related to product) may be established, on the acknowledgments page, so long as disclaimers are clearly in place, indicating that Hamad Medical Corporation/ DHP is not responsible for the linked content.
3.16 Printed CPD Handouts or Syllabi, Advertisements, Promotional Material:
3.16.1
Advertisement and promotional materials should not be inserted within the pages of the CPD content.
They may be inserted at the end of the syllabus, not facing any content, and should be clearly marked as advertising or promotional content.
3.17 Live Activities:
3.17.1 Commercial displays and materials should be in a
separate room from educational activities. Providers may not allow representatives of commercial interests to engage in sales or promotional activities during the accredited program. In acknowledgement of commercial sponsors, e.g. presentation slides or announcements, only the company name may be used. The use of product names is strictly prohibited.
3.18 Sponsorship Agreements:
3.18.1 There should be a signed agreement when any monetary or in kind sponsorship is engaged in (Toolkit)
and this should be included in the application for CPD accreditation of the event.
4.0 DOCUMENTIONS: Not Applicable.
5.0 REFERENCES:
5.1 DHP Accreditation Standards.
5.2 OP4071 Sponsorship by Pharmaceutical Companies.
5.3 OP 4011 Conflict of Interest policy.
5.4 Privacy and Confidentiality policy
6.0 ATTACHMENTS:
6.1 Sponsorship Agreement Form. See
Toolkit
6.2
HMC Sponsorship Procedure. See
Toolkit
6.3  OP 4041 Approval of Paid Accredited Continuing Professional Development (CPD) Activities
OP 4041 Approval of Paid Accredited Continuing Professional Development (CPD) Activities